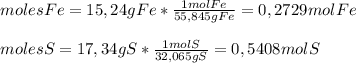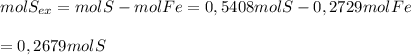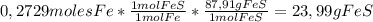Chemistry, 24.07.2019 08:30 tylervreeland022202
Sulfur combines with iron to form iron(ii) sulfide. in an experiment, 15.24 g of fe are allowed to react with 17.34 g of sulfur what is the limiting reactant? the reactant in excess? what mass of fes is created?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Sulfur combines with iron to form iron(ii) sulfide. in an experiment, 15.24 g of fe are allowed to r...
Questions




Biology, 07.12.2020 22:00




Mathematics, 07.12.2020 22:00



History, 07.12.2020 22:00


Advanced Placement (AP), 07.12.2020 22:00


Advanced Placement (AP), 07.12.2020 22:00

Social Studies, 07.12.2020 22:00

Mathematics, 07.12.2020 22:00

Mathematics, 07.12.2020 22:00

Mathematics, 07.12.2020 22:00