
Chemistry, 24.07.2019 11:30 AshlynPlayz45
In 1909 fritz haber discovered the workable conditions under which nitrogen, n2(g), and hydrogen, h2(g), would combine using to produce ammonia. the conditions included medium temperature (~500oc), very high pressure (~351kpa), and an iron catalyst. the reaction is represented by the equation: n2(g) + 3h2(g) → 2nh3(g) how many grams of nitrogen are needed to produce 100 grams of ammonia gas?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 21.06.2019 15:30
If 200.0g of copper(ll) sulfate react with an excess of zinc metal, what is the theoretical yield of copper
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2
You know the right answer?
In 1909 fritz haber discovered the workable conditions under which nitrogen, n2(g), and hydrogen, h2...
Questions



History, 01.08.2019 13:47

Biology, 01.08.2019 13:47


Spanish, 01.08.2019 13:47






English, 01.08.2019 13:47








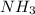 = 100 g
= 100 g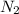 = 28 g/mole
= 28 g/mole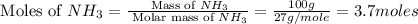
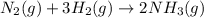
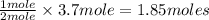 of
of 

