
Chemistry, 24.07.2019 12:30 jayowens20
For the reaction x2 + y + z → xy + xz, it is found that doubling the concentration of x2 doubles the reaction rate, tripling the concentration of y triples the rate, and doubling the concentration of z has no effect. what is the rate law for this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2
You know the right answer?
For the reaction x2 + y + z → xy + xz, it is found that doubling the concentration of x2 doubles the...
Questions

Mathematics, 30.11.2020 20:00

English, 30.11.2020 20:00


Mathematics, 30.11.2020 20:00



Mathematics, 30.11.2020 20:00


Mathematics, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00




Mathematics, 30.11.2020 20:00



Mathematics, 30.11.2020 20:00


Health, 30.11.2020 20:00

![\boxed{rate=k\left[ {{{\text{X}}_2}}\right]\left[ {\text{Y}} \right]}](/tpl/images/0127/2448/0a486.png) .
.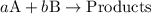
![{\text{rate}}=k{\left[{\text{A}}\right]^a}{\left[{\text{B}}\right]^b}](/tpl/images/0127/2448/dedd1.png) ...... (1)
...... (1)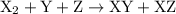
![{\text{rate}}=k{\left[{{{\text{X}}_2}}\right]^a}{\left[{\text{Y}}\right]^b}{\left[ {\text{Z}} \right]^c}](/tpl/images/0127/2448/0b279.png) …… (2)
…… (2) , Y, and Z respectively.
, Y, and Z respectively.![\begin{aligned}{\text{rate}}&=k{\left[{{{\text{X}}_2}}\right]^a}{\left[{\text{Y}}\right]^b}{\left[ {\text{Z}}\right]^c}\\&=k{\left[{{{\text{X}}_2}}\right]^1}{\left[ {\text{Y}}\right]^1}{\left[ {\text{Z}}\right]^0}\\&=k\left[{{{\text{X}}_2}}\right]\left[ {\text{Y}}\right]\\\end{aligned}](/tpl/images/0127/2448/fee78.png)


