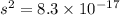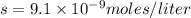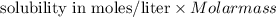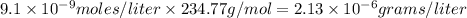Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
Calculate the molar solubility and the solubility in g / l of agi at 25°c. the ksp of agi is 8.3 × 1...
Questions


Biology, 15.03.2022 07:30


Mathematics, 15.03.2022 07:30

Mathematics, 15.03.2022 07:40



Mathematics, 15.03.2022 07:40

Mathematics, 15.03.2022 07:40

Mathematics, 15.03.2022 07:40






Mathematics, 15.03.2022 07:40

Mathematics, 15.03.2022 07:40

Mathematics, 15.03.2022 07:40

Computers and Technology, 15.03.2022 07:40

Mathematics, 15.03.2022 07:40

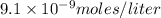 and solubility in grams per liter is
and solubility in grams per liter is 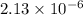
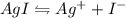
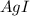 gives 1 mole of
gives 1 mole of 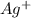 and 1 mole of
and 1 mole of 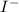 .
.![K_sp=[Ag^+][I^-]](/tpl/images/0128/0319/f6f6c.png)
![8.3\times 10^{-17}=[s][s]](/tpl/images/0128/0319/983c8.png)