
Chemistry, 24.07.2019 22:00 Haileydusenbery
6tons of pig ovaries were required to extract 13.0 mg of estrone. assuming complete isolation of the hormones was achieved, calculate the mass percentage of estrone in the original tissue.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

You know the right answer?
6tons of pig ovaries were required to extract 13.0 mg of estrone. assuming complete isolation of the...
Questions

Mathematics, 13.07.2019 09:20


Social Studies, 13.07.2019 09:20


History, 13.07.2019 09:20

Mathematics, 13.07.2019 09:20

History, 13.07.2019 09:20

Mathematics, 13.07.2019 09:30

Mathematics, 13.07.2019 09:30

Mathematics, 13.07.2019 09:30

History, 13.07.2019 09:30

Mathematics, 13.07.2019 09:30

Biology, 13.07.2019 09:30


Mathematics, 13.07.2019 09:30

Mathematics, 13.07.2019 09:30

English, 13.07.2019 09:30

Mathematics, 13.07.2019 09:30

Physics, 13.07.2019 09:30

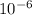 %
%


