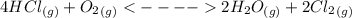1. what would happen to the position of equilibrium when the following changes are made to the reaction below? 2hg3o (g) ↔ 6hg (g) + o2 (g) δh- (a) hg3o is added to the system (b) the volume of the system decreases (c) temperature is increased 2. balance the exothermic reaction no2 ↔ n2o4 kj – 58.0kj predict the effect of each of the following changes on this system at equilibrium (a) added n2o4 (b) increase the volume (c) add n2 (d) remove no2 (e) decrease the temperature 3. + 333 kj 2no2 ↔ n2 + o2 (a) no2 is added to the system (b) n2 is added to the system (c) o2 is removed from the system (d) the temperature of the container increased (e) the volume of the container increase (f) n2 is added and no2 is removed 4. 179 kj caco3 ↔ cao + co2 (a) co2 is added (b) the volume of the container decreased (c) cao is removed from the system (d) the temperature of the container decreased (e) the volume of the container is increased. (f) caco3 is added to the system 5. h2 + cl2 ↔ 2hcl (a) h2 is removed from the system (b) hcl is removed from the system (c) the volume of the container is removed (d) the temperature of the container is increased (e) the concentration of cl2 is decreased (f) the volume of the container decreased 6. list all the “stressors” that could be applied to this equilibrium reaction, which would cause an increase in the concentration of water vapor. 4hcl (g) + o2 (g) ↔ 2h2o (g) + 2cl2 (g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1


Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
1. what would happen to the position of equilibrium when the following changes are made to the react...
Questions

Mathematics, 24.11.2019 10:31


History, 24.11.2019 10:31

Social Studies, 24.11.2019 10:31

Spanish, 24.11.2019 10:31


Physics, 24.11.2019 10:31










Mathematics, 24.11.2019 10:31


Biology, 24.11.2019 10:31

History, 24.11.2019 10:31


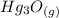 is 272.1 KJ/mol & std heat of formation of Hg is 61.38 KJ/mol,
is 272.1 KJ/mol & std heat of formation of Hg is 61.38 KJ/mol, 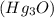
 .
. 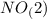 will give 1 dinitrogen tetraoxide.
will give 1 dinitrogen tetraoxide. 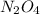 is added to the rxn the rxn eqm will be shifted to <--.
is added to the rxn the rxn eqm will be shifted to <--. 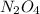 will Incr. the prod & so it will shift the eqm to the backward rxn to produce more reactants.
will Incr. the prod & so it will shift the eqm to the backward rxn to produce more reactants. 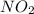 the eqm will shift to <--.
the eqm will shift to <--.  balanced rxn.
balanced rxn. 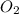 is removed from the rxn then eqm will shift on -->.
is removed from the rxn then eqm will shift on -->. 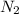 is added &
is added &  balanced equation. Endo rxn as ΔH is +ve = 179 KJ.
balanced equation. Endo rxn as ΔH is +ve = 179 KJ. 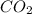 is added to the rxn then the eqm shifts to <--.
is added to the rxn then the eqm shifts to <--. 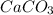 is added will shift to -->.
is added will shift to -->.  It is the balanced equation & is exo rxn.
It is the balanced equation & is exo rxn.