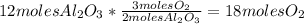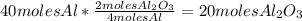Chemistry, 25.07.2019 01:00 ethanhose05
Balance this reaction: al(s) + o2(g) → al2o3(s) how many moles of oxygen will be needed to react with aluminum metal to produce 12.0 moles of aluminum oxide? 6. using the reaction listed in question 5 (above), how many moles of aluminum oxide will be produced by 40.0 moles of aluminum reacting completely with a boat load (that is a lot) of oxygen?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1
You know the right answer?
Balance this reaction: al(s) + o2(g) → al2o3(s) how many moles of oxygen will be needed to react w...
Questions

Mathematics, 13.12.2020 22:00


Mathematics, 13.12.2020 22:10

Biology, 13.12.2020 22:10



History, 13.12.2020 22:10


Chemistry, 13.12.2020 22:10

English, 13.12.2020 22:10



History, 13.12.2020 22:10