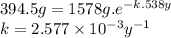Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Asample of argon-39 had an original mass of 1578 grams. after 538 years, the sample is 394.5 grams....
Questions




Mathematics, 08.07.2019 03:30


Social Studies, 08.07.2019 03:30


History, 08.07.2019 03:30

Chemistry, 08.07.2019 03:30


Mathematics, 08.07.2019 03:30



Mathematics, 08.07.2019 03:30

Chemistry, 08.07.2019 03:30



World Languages, 08.07.2019 03:30

Physics, 08.07.2019 03:30

Social Studies, 08.07.2019 03:30

![[Ar]_{t}=[Ar]_{0}.e^{-k.t}](/tpl/images/0129/2552/6cc5e.png)
![[Ar]_{t}](/tpl/images/0129/2552/de21b.png) is the amount of Ar at a certain time t
is the amount of Ar at a certain time t![[Ar]_{0}](/tpl/images/0129/2552/3b0e7.png) is the initial amount of Ar
is the initial amount of Ar