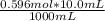Chemistry, 25.07.2019 01:30 officialrogerfp3gf2s
How many grams of pbcl2 are formed when 10.0 ml of 0.596 m kcl react with pb(no3)2?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

You know the right answer?
How many grams of pbcl2 are formed when 10.0 ml of 0.596 m kcl react with pb(no3)2?...
Questions

Engineering, 04.12.2020 08:20

Mathematics, 04.12.2020 08:20

Chemistry, 04.12.2020 08:20

Mathematics, 04.12.2020 08:20

Arts, 04.12.2020 08:20

Mathematics, 04.12.2020 08:20

English, 04.12.2020 08:20

Mathematics, 04.12.2020 08:20




Chemistry, 04.12.2020 08:20

Chemistry, 04.12.2020 08:20


Social Studies, 04.12.2020 08:20


Health, 04.12.2020 08:20

Biology, 04.12.2020 08:20


Mathematics, 04.12.2020 08:20