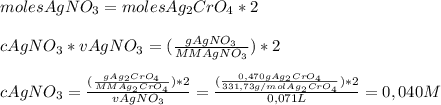We add excess na2cro4 solution to 71.0 ml of a solution of silver nitrate (agno3) to form insoluble solid ag2cro4. when it has been dried and weighed, the mass of ag2cro4 is found to be 0.470 grams. what is the molarity of the agno3 solution? answer in units of m.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2
You know the right answer?
We add excess na2cro4 solution to 71.0 ml of a solution of silver nitrate (agno3) to form insoluble...
Questions



History, 29.01.2020 03:59

History, 29.01.2020 03:59


Mathematics, 29.01.2020 03:59

Mathematics, 29.01.2020 03:59


Mathematics, 29.01.2020 03:59


Mathematics, 29.01.2020 03:59



Mathematics, 29.01.2020 03:59

Computers and Technology, 29.01.2020 03:59


History, 29.01.2020 03:59


Biology, 29.01.2020 03:59

Chemistry, 29.01.2020 03:59