Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2
You know the right answer?
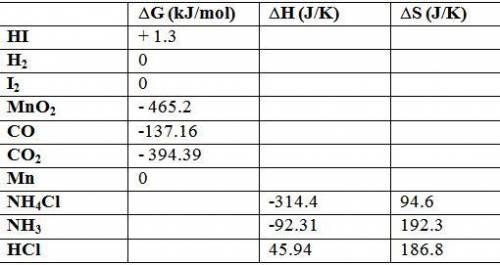
Calculate δg o for each reaction using δg of values: (a) h2(g) + i2(s) → 2hi(g) kj (b) mno2(s) + 2co...
Questions



Biology, 09.03.2020 12:23

English, 09.03.2020 12:24




Mathematics, 09.03.2020 12:28

Mathematics, 09.03.2020 12:29

Mathematics, 09.03.2020 12:32

Computers and Technology, 09.03.2020 12:34

Mathematics, 09.03.2020 12:36

Social Studies, 09.03.2020 12:37

Mathematics, 09.03.2020 12:37

History, 09.03.2020 12:40


Mathematics, 09.03.2020 12:40

Biology, 09.03.2020 12:40


English, 09.03.2020 12:43