
Chemistry, 25.07.2019 07:30 ramentome7542
When a 3.80 g sample of magnesium nitride (mw 101g/mol) is reacted with 3.30 g of water, 3.60 g of mgo is formed. what is the percent yield of this reaction? mg3n2 + 3 h2o --> 2 nh3 + 3 mgo

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

You know the right answer?
When a 3.80 g sample of magnesium nitride (mw 101g/mol) is reacted with 3.30 g of water, 3.60 g of m...
Questions

Mathematics, 20.11.2020 19:50

Social Studies, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

Health, 20.11.2020 19:50



Mathematics, 20.11.2020 19:50







Spanish, 20.11.2020 19:50



Health, 20.11.2020 19:50

English, 20.11.2020 19:50


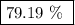 .
.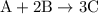
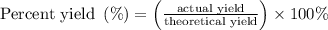 ......(1)
......(1)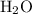 and is as follows:
and is as follows:
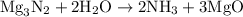 ……. (2)
……. (2)
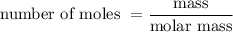
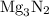 is
is 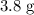 .
.
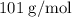 .
.
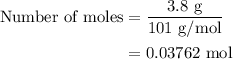
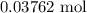 .
.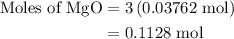
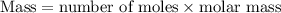
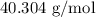 .
.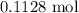 .
.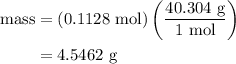
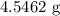 .
.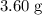 .
.
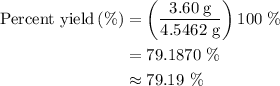
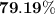 .
.



