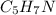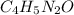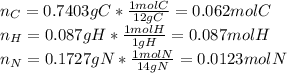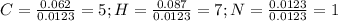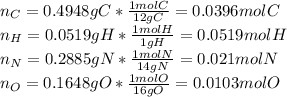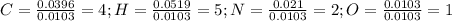Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
Calculate the empirical formula for each stimulant based on its elemental mass percent composition....
Questions






Chemistry, 11.11.2021 03:00





Social Studies, 11.11.2021 03:00

Mathematics, 11.11.2021 03:00



Mathematics, 11.11.2021 03:00


English, 11.11.2021 03:00


Mathematics, 11.11.2021 03:00

Mathematics, 11.11.2021 03:00