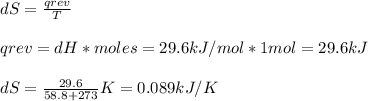Chemistry, 25.07.2019 11:00 kornut7316
The normal boiling point of bromine, br2(l), is 58.8°c, and its molar enthalpy of vaporization is δhvap = 29.6 kj/mol. (a) when br2(l) boils at its normal boiling point, does its entropy increase or decrease? decrease (δs is negative) increase (δs is positive) (b) calculate the value of δs when 1.00 mol of br2(l) is vaporized at 58.8°c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2
You know the right answer?
The normal boiling point of bromine, br2(l), is 58.8°c, and its molar enthalpy of vaporization is δh...
Questions

History, 07.10.2019 04:30


Chemistry, 07.10.2019 04:30

Mathematics, 07.10.2019 04:30



History, 07.10.2019 04:30


English, 07.10.2019 04:30


Mathematics, 07.10.2019 04:30

English, 07.10.2019 04:30







History, 07.10.2019 04:30