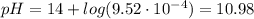Chemistry, 25.07.2019 16:00 Tyrant4life
For the titration of 50.0 ml of 0.020 m aqueous salicylic acid with 0.020 m koh (aq), calculate the ph after the addition of 55.0 ml of the base. for salycylic acid, pka = 2.97.'

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2

You know the right answer?
For the titration of 50.0 ml of 0.020 m aqueous salicylic acid with 0.020 m koh (aq), calculate the...
Questions

English, 31.03.2021 19:40

Geography, 31.03.2021 19:40


Social Studies, 31.03.2021 19:40

History, 31.03.2021 19:40

Mathematics, 31.03.2021 19:40



Mathematics, 31.03.2021 19:40

History, 31.03.2021 19:40

Mathematics, 31.03.2021 19:40

Social Studies, 31.03.2021 19:40


Geography, 31.03.2021 19:40





Mathematics, 31.03.2021 19:40

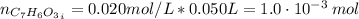
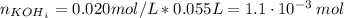
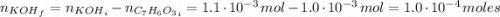
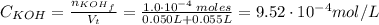
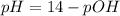
![pH = 14 - (-log[OH^{-}])](/tpl/images/0131/6319/7f1c5.png)