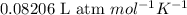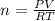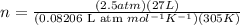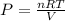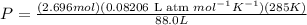Chemistry, 25.07.2019 18:00 reese12345
Find the total pressure of a gas that initially occupied 27 l at 32 degrees celsius and 2.5 atm, if the final conditions are 12 degrees celsius and 88.0 l

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1


You know the right answer?
Find the total pressure of a gas that initially occupied 27 l at 32 degrees celsius and 2.5 atm, if...
Questions


Physics, 21.11.2020 01:00

History, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00



Mathematics, 21.11.2020 01:00

Geography, 21.11.2020 01:00

Biology, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00



Biology, 21.11.2020 01:00

History, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Biology, 21.11.2020 01:00


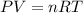 .......(1)
.......(1)