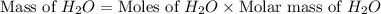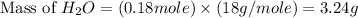Gaseous methane ch4 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 1.44 g of methane is mixed with 9.5 g of oxygen. calculate the maximum mass of water that could be produced by the chemical reaction. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

You know the right answer?
Gaseous methane ch4 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gase...
Questions

Mathematics, 28.05.2021 14:00

English, 28.05.2021 14:00

Mathematics, 28.05.2021 14:00



Mathematics, 28.05.2021 14:00

Mathematics, 28.05.2021 14:00

Geography, 28.05.2021 14:00

Mathematics, 28.05.2021 14:00

Mathematics, 28.05.2021 14:00

Biology, 28.05.2021 14:00

Chemistry, 28.05.2021 14:00

Mathematics, 28.05.2021 14:00


Geography, 28.05.2021 14:00



Mathematics, 28.05.2021 14:00


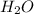 produced will be, 3.24 grams
produced will be, 3.24 grams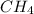 = 1.44 g
= 1.44 g
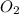 = 9.5 g
= 9.5 g
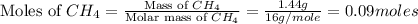
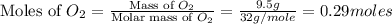
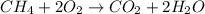
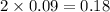 moles of
moles of