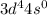Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
Am2+ ion has four electrons in the 3d subshell and is derived from a metal in the first transition m...
Questions


Mathematics, 11.04.2020 06:18


English, 11.04.2020 06:18

Mathematics, 11.04.2020 06:18


Mathematics, 11.04.2020 06:18

Mathematics, 11.04.2020 06:19


Biology, 11.04.2020 06:19

English, 11.04.2020 06:19



Mathematics, 11.04.2020 06:19

History, 11.04.2020 06:19

Mathematics, 11.04.2020 06:19

Mathematics, 11.04.2020 06:19


English, 11.04.2020 06:19

English, 11.04.2020 06:20

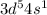
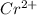 : [Ar]
: [Ar]