
Chemistry, 26.09.2019 01:00 fickllyd000
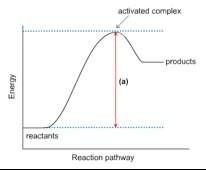
1. this energy diagram is for the thermal decomposition of solid mercury (ii) oxide (also known as mercuric oxide) into liquid mercury and oxygen gas.
• write a balanced equation for the reaction.
• explain what feature is shown by the arrow labeled (a).
• using chemical symbols and dashed lines (this can be done with type), draw what the activated complex or transition state might look like.
• is this reaction exothermic or endothermic? explain.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
1. this energy diagram is for the thermal decomposition of solid mercury (ii) oxide (also known as m...
Questions




Social Studies, 06.11.2020 21:10

English, 06.11.2020 21:10





Mathematics, 06.11.2020 21:10

History, 06.11.2020 21:10


Mathematics, 06.11.2020 21:10



Mathematics, 06.11.2020 21:10

Biology, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10



