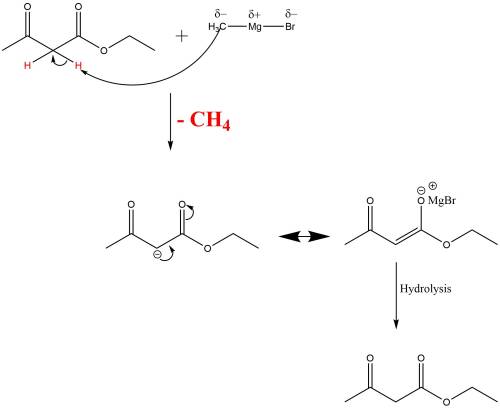
When ethyl acetoacetate (ch3coch2co2ch2ch3) is treated with one equivalent of ch3mgbr, a gas is evolved from the reaction mixture, and after adding aqueous acid, ethyl acetoacetate is recovered in high yield. identify the gas formed and explain why the starting material was recovered in this reaction. be sure to answer all parts?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2
You know the right answer?
When ethyl acetoacetate (ch3coch2co2ch2ch3) is treated with one equivalent of ch3mgbr, a gas is evol...
Questions

Mathematics, 30.08.2021 14:00

Mathematics, 30.08.2021 14:00


Business, 30.08.2021 14:00


Biology, 30.08.2021 14:00

Chemistry, 30.08.2021 14:00


Computers and Technology, 30.08.2021 14:00

Mathematics, 30.08.2021 14:00



Computers and Technology, 30.08.2021 14:00

Mathematics, 30.08.2021 14:00




Mathematics, 30.08.2021 14:00


Social Studies, 30.08.2021 14:00