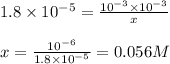Chemistry, 26.07.2019 15:00 lucerogon7403
Determine the ammonia concentration of an aqueous solution that has a ph of 11.00. the equation for the dissociation of nh3 (kb = 1.8 × 10-5) is determine the ammonia concentration of an aqueous solution that has a ph of 11.00. the equation for the dissociation of nh3 (kb = 1.8 × 10-5) is 1.8 × 10-2 m

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
Determine the ammonia concentration of an aqueous solution that has a ph of 11.00. the equation for...
Questions

Mathematics, 03.08.2019 01:00





Business, 03.08.2019 01:00

Mathematics, 03.08.2019 01:00


Health, 03.08.2019 01:00


Social Studies, 03.08.2019 01:00

History, 03.08.2019 01:00





History, 03.08.2019 01:00

Geography, 03.08.2019 01:00


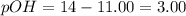
![pOH=-\log[OH^-]](/tpl/images/0135/3145/fe336.png)
![3.00=-\log [OH^-]](/tpl/images/0135/3145/bf5a2.png)
![[OH^-]=10^{-3}M](/tpl/images/0135/3145/d9858.png)
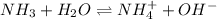
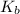 for above equation follows:
for above equation follows:![K_b=\frac{[NH_4^+][OH^-]}{[NH_3]}](/tpl/images/0135/3145/00f50.png)
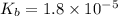
![[NH_4^+]=[OH^-]=10^{-3}M](/tpl/images/0135/3145/b4c85.png)