Chemistry, 26.07.2019 15:00 victorialeona81
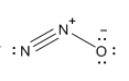
Which resonance form is likely to contribute most to the correct structure of n2o? which resonance form is likely to contribute most to the correct structure of ? structure for nno showing three lone-pairs of electrons on the terminal nitrogen atom, a single bond between the two nitrogen atoms, a triple bond between nitrogen and oxygen, and one lone-pair of electrons on the terminal oxygen atom. structure for nno showing two lone-pairs of electrons on the terminal nitrogen atom, a double bond between the two nitrogen atoms, a double bond between nitrogen and oxygen, and two lone-pairs of electrons on the terminal oxygen atom. structure for nno showing one lone-pair of electrons on the terminal nitrogen atom, a triple bond between the two nitrogen atoms, a single bond between nitrogen and oxygen, and three lone-pairs of electrons on the terminal oxygen atom?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1
You know the right answer?
Which resonance form is likely to contribute most to the correct structure of n2o? which resonance...
Questions



Mathematics, 13.10.2019 13:20

Mathematics, 13.10.2019 13:20

Mathematics, 13.10.2019 13:20


Social Studies, 13.10.2019 13:20

Mathematics, 13.10.2019 13:20


Health, 13.10.2019 13:20

History, 13.10.2019 13:20


History, 13.10.2019 13:20

History, 13.10.2019 13:20

History, 13.10.2019 13:20


Social Studies, 13.10.2019 13:20

Chemistry, 13.10.2019 13:20

Geography, 13.10.2019 13:20

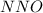 is:
is: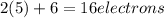
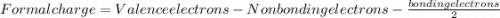
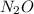 is:
is: