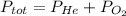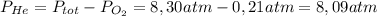He partial pressure of oxygen gas in our atmosphere is 0.21 atm. this is the partial pressure at which human lungs have evolved to be able to breathe this gas. a scuba diver, will thus still have to breath oxygen at this pressure even when diving way down in the water. if a mixture of helium and oxygen (heliox) in his tank is at a pressure of 8.30 atm, what must the partial pressure be of helium to keep the partial pressure of oxygen at 0.21 atm?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
He partial pressure of oxygen gas in our atmosphere is 0.21 atm. this is the partial pressure at whi...
Questions

Mathematics, 09.06.2021 20:10

Mathematics, 09.06.2021 20:10


English, 09.06.2021 20:10

Chemistry, 09.06.2021 20:10

Mathematics, 09.06.2021 20:10

Chemistry, 09.06.2021 20:10


Mathematics, 09.06.2021 20:10


Health, 09.06.2021 20:10

Mathematics, 09.06.2021 20:10

Physics, 09.06.2021 20:10



Mathematics, 09.06.2021 20:10


English, 09.06.2021 20:10

Mathematics, 09.06.2021 20:10