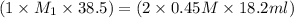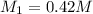If it requires 18.2 milliliters of 0.45 molar barium hydroxide to neutralize 38.5 milliliters of nitric acid, solve for the molarity of the nitric acid solution. show all of the work used to solve this problem. unbalanced equation: ba(oh)2 + hno3 yields ba(no3)2 + h2o

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
If it requires 18.2 milliliters of 0.45 molar barium hydroxide to neutralize 38.5 milliliters of nit...
Questions

History, 16.10.2019 14:50

Health, 16.10.2019 14:50




History, 16.10.2019 14:50


Mathematics, 16.10.2019 14:50



Geography, 16.10.2019 14:50


Mathematics, 16.10.2019 14:50

Mathematics, 16.10.2019 14:50

Mathematics, 16.10.2019 14:50



Health, 16.10.2019 14:50

Mathematics, 16.10.2019 14:50

Chemistry, 16.10.2019 14:50

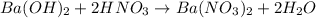
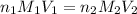
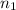 = basicity of
= basicity of 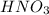 = 1
= 1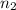 = acidity of
= acidity of 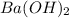 = 2
= 2
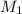 = concentration of
= concentration of 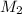 = concentration of
= concentration of 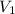 = volume of
= volume of 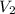 = volume of
= volume of