
Chemistry, 27.07.2019 05:30 SoccerHalo
The theoretical yield for a reaction is 55.9 g licl. the actual yield is 24.6 g licl. what is the percent yield of the reaction? question options: 227% 44% 25% 56%

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

You know the right answer?
The theoretical yield for a reaction is 55.9 g licl. the actual yield is 24.6 g licl. what is the pe...
Questions










Chemistry, 20.12.2020 17:00



Mathematics, 20.12.2020 17:00



English, 20.12.2020 17:00

Mathematics, 20.12.2020 17:00


Geography, 20.12.2020 17:00

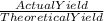 × 100
× 100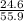 × 100
× 100

