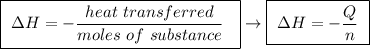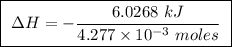Chemistry, 27.07.2019 07:00 alejandra216
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.120-g sample of ethylene (c2h4) was burned in this calorimeter, the temperature increased by 2.44 k. calculate the enthalpy change per mole of ethylene combusted.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which of the following can be used to measure electricity
Answers: 1

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1
You know the right answer?
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.120-g sample of ethylene (c2h4) was bur...
Questions

History, 28.07.2019 12:30


Mathematics, 28.07.2019 12:30

History, 28.07.2019 12:30

Mathematics, 28.07.2019 12:30



Mathematics, 28.07.2019 12:30


Biology, 28.07.2019 12:30

Mathematics, 28.07.2019 12:30

History, 28.07.2019 12:30

Mathematics, 28.07.2019 12:30

History, 28.07.2019 12:30


English, 28.07.2019 12:30


Mathematics, 28.07.2019 12:30

Biology, 28.07.2019 12:30

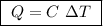
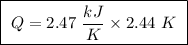
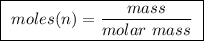
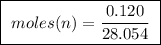
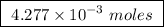 of ethylene.
of ethylene.