Chemistry, 27.07.2019 08:30 xxxamslashxxx9
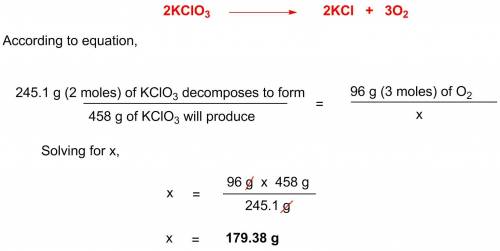
Potassium chlorate decomposes according to the following chemical equation: 2kclo3 > 2kcl + 3o2, if you start with 458 g of kclo3, what mass (g) of o2 will be produced? a. 83.71 l o2 b. 179.39 l o2 c. 687 l o2 d. 67.2 l o2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1
You know the right answer?
Potassium chlorate decomposes according to the following chemical equation: 2kclo3 > 2kcl + 3o2...
Questions

Mathematics, 20.07.2019 00:30



Chemistry, 20.07.2019 00:30


History, 20.07.2019 00:30




Social Studies, 20.07.2019 00:30




Biology, 20.07.2019 00:30




Social Studies, 20.07.2019 00:30