Chemistry, 27.07.2019 10:30 FlayMaster101
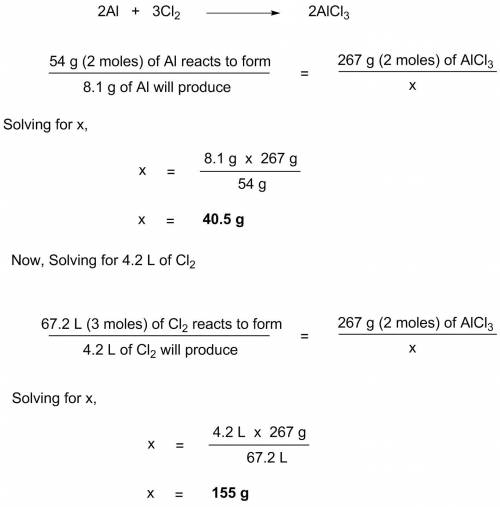
What mass of aluminum chloride could be made from 8.1 g of aluminum and 4.2 l of chlorine at stp?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1
You know the right answer?
What mass of aluminum chloride could be made from 8.1 g of aluminum and 4.2 l of chlorine at stp?...
Questions





Mathematics, 25.03.2020 21:00

Biology, 25.03.2020 21:00



Mathematics, 25.03.2020 21:00



Spanish, 25.03.2020 21:00



Biology, 25.03.2020 21:00

Mathematics, 25.03.2020 21:00


Social Studies, 25.03.2020 21:00

Mathematics, 25.03.2020 21:00

History, 25.03.2020 21:00