

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1

Chemistry, 23.06.2019 12:30
Atriple covalent bond involves two atoms sharing three pairs of electrons. true false
Answers: 2
You know the right answer?
The nickel(ii) ion is commonly dissolved in solution using nickel(ii) nitrate hexahydrate, ni(no3)2....
Questions



English, 05.05.2021 04:40


History, 05.05.2021 04:40


Mathematics, 05.05.2021 04:40

Mathematics, 05.05.2021 04:40

Mathematics, 05.05.2021 04:40

Mathematics, 05.05.2021 04:40

Mathematics, 05.05.2021 04:40

Computers and Technology, 05.05.2021 04:40


Mathematics, 05.05.2021 04:40


Arts, 05.05.2021 04:40

Mathematics, 05.05.2021 04:40



English, 05.05.2021 04:40

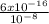 = 6 x 10⁻⁸ M
= 6 x 10⁻⁸ M


