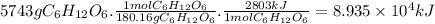Chemistry, 27.07.2019 13:00 rakanmadi87
Given the thermochemical equation for photosynthesis, 6h2o(l) + 6co2(g) → c6h12o6(s) + 6o2(g) δh = +2803 kj/mol calculate the solar energy required to produce 5743 g of c6h12o6.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1


Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 08:00
Amechanical wave that transports a lot of energy will have a
Answers: 2
You know the right answer?
Given the thermochemical equation for photosynthesis, 6h2o(l) + 6co2(g) → c6h12o6(s) + 6o2(g) δh = +...
Questions

Mathematics, 18.04.2020 22:13


Biology, 18.04.2020 22:13


Chemistry, 18.04.2020 22:14




Mathematics, 18.04.2020 22:14





Law, 18.04.2020 22:14

Mathematics, 18.04.2020 22:14

Chemistry, 18.04.2020 22:14

Spanish, 18.04.2020 22:14

Mathematics, 18.04.2020 22:14

Mathematics, 18.04.2020 22:15