
Chemistry, 16.01.2020 05:31 skylerdemi1
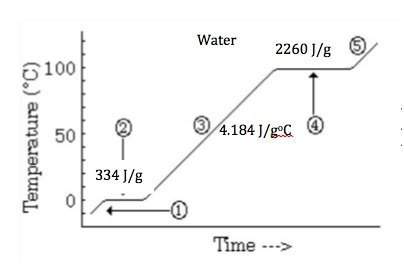
Elaborate the reason that the heat of vaporization of water is so much greater than its heat of fusion.
a) the potential energy of fusion represents a greater change of stored energy.
b) liquid water is stabilized by strong intermolecular forces between the molecules.
c) the kinetic energy of the water molecules is so much greater in the liquid state.
d) the bonding forces for solid water are much stronger than the bonding in the liquid.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
Elaborate the reason that the heat of vaporization of water is so much greater than its heat of fusi...
Questions

Mathematics, 27.05.2021 05:30

Mathematics, 27.05.2021 05:30


English, 27.05.2021 05:30

Mathematics, 27.05.2021 05:30

Mathematics, 27.05.2021 05:30



Mathematics, 27.05.2021 05:30


Chemistry, 27.05.2021 05:30

Mathematics, 27.05.2021 05:30


History, 27.05.2021 05:30


Mathematics, 27.05.2021 05:30

Mathematics, 27.05.2021 05:30

Physics, 27.05.2021 05:30




