Chemistry, 27.07.2019 17:30 nockturnal1993
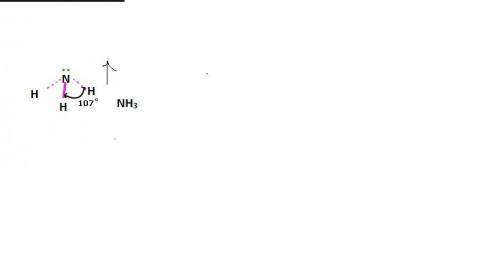
In ammonia, a central nitrogen atom is bonded to three hydrogen atoms. similarly, boron trifluoride has a central boron atom bonded to three fluorine atoms. however, ammonia is pyramidal and boron trifluoride is trigonal planar in shape. which statement justifies this difference in their structure? the lone pair of electrons in boron trifluoride increases the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in boron trifluoride decreases the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in boron trifluoride stabilizes the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in ammonia increases the bond angle between the bonding pairs, giving it a pyramidal structure. the lone pair of electrons on the nitrogen in ammonia decreases the bond angle between the bonding pairs, giving it a pyramidal structure.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

You know the right answer?
In ammonia, a central nitrogen atom is bonded to three hydrogen atoms. similarly, boron trifluoride...
Questions

Spanish, 03.12.2019 14:31


Biology, 03.12.2019 14:31



History, 03.12.2019 14:31


Mathematics, 03.12.2019 14:31

Social Studies, 03.12.2019 14:31


Mathematics, 03.12.2019 14:31

Mathematics, 03.12.2019 14:31


Mathematics, 03.12.2019 15:31

Social Studies, 03.12.2019 15:31

Social Studies, 03.12.2019 15:31


Social Studies, 03.12.2019 15:31


![:{\text{Number of electrons}} =\frac{1}{2}[V+N-C+A]](/tpl/images/0139/5742/08b6a.png)
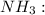
![{\text{Number of electrons}} =\frac{1}{2}[5+3-0+0]=4](/tpl/images/0139/5742/46c6e.png)
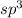 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.
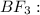
![{\text{Number of electrons}} =\frac{1}{2}[3+3-0+0]=3](/tpl/images/0139/5742/beb45.png)
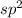 and the electronic geometry of the molecule will be trigonal planar.
and the electronic geometry of the molecule will be trigonal planar.