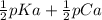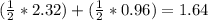Chemistry, 28.07.2019 07:00 hewonabi123
Saccharin, a sugar substitute, is a weak acid with pka=2.32 at 25 ∘c. it ionizes in aqueous solution as follows: hnc7h4so3(aq)←−→h+(aq)+nc7h4so−3(aq ) part a what is the ph of a 0.11 m solution of this substance?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
Saccharin, a sugar substitute, is a weak acid with pka=2.32 at 25 ∘c. it ionizes in aqueous solution...
Questions

Computers and Technology, 31.07.2019 05:30


Chemistry, 31.07.2019 05:30


Mathematics, 31.07.2019 05:30



English, 31.07.2019 05:30


History, 31.07.2019 05:30

History, 31.07.2019 05:30

Mathematics, 31.07.2019 05:30





Mathematics, 31.07.2019 05:30