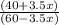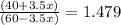Chemistry, 28.07.2019 07:00 kitkat033157
Suppose you have 1.00 l of an aqueous buffer containing 60.0 mmol acetic acid (pka = 4.76) and 40.0 mmol acetate. what volume of 3.50 m naoh would be required to increase the ph to 4.93?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 06:00
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
You know the right answer?
Suppose you have 1.00 l of an aqueous buffer containing 60.0 mmol acetic acid (pka = 4.76) and 40.0...
Questions

Mathematics, 04.02.2021 20:50

Mathematics, 04.02.2021 20:50












Mathematics, 04.02.2021 20:50


Mathematics, 04.02.2021 20:50


Chemistry, 04.02.2021 20:50


Computers and Technology, 04.02.2021 20:50

![\frac{[CH_{3}COO^-]}{[CH_3COOH]}](/tpl/images/0141/9497/e74fb.png)