
In class, students were given the following problem: "aluminum satellite dishes resist corrosion because aluminum reacts with oxygen gas to form a coating of aluminum oxide (al2o3). what is the mass of al2o3? "
a) sally solved the problem by first finding the molar mass of aluminum oxide. she calculated the molar mass of al2o3 as 43.0 g/mol. this is not the correct molar mass however. what did sally do wrong?
b) after finding the molar mass of al2o3, jacob set up the following conversion. (i'll add a pic)
is this the correct set up for the problem?
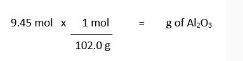

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
In class, students were given the following problem: "aluminum satellite dishes resist corrosion be...
Questions

English, 06.01.2021 06:30


Mathematics, 06.01.2021 06:30

Spanish, 06.01.2021 06:30


Mathematics, 06.01.2021 06:30



Social Studies, 06.01.2021 06:30


Mathematics, 06.01.2021 06:30



Chemistry, 06.01.2021 06:30

Mathematics, 06.01.2021 06:30

English, 06.01.2021 06:30


Spanish, 06.01.2021 06:30

Mathematics, 06.01.2021 06:30

Mathematics, 06.01.2021 06:30



