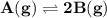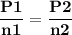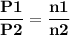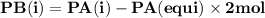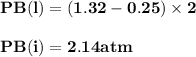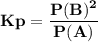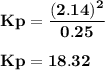Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1
You know the right answer?
For the reaction a(g) 2 b(g), a reaction vessel initially contains only a at a pressure of pa = 1.32...
Questions




Mathematics, 08.01.2021 16:30

Mathematics, 08.01.2021 16:30

Biology, 08.01.2021 16:30


English, 08.01.2021 16:30



Mathematics, 08.01.2021 16:30

Chemistry, 08.01.2021 16:30

Mathematics, 08.01.2021 16:30



English, 08.01.2021 16:30


Medicine, 08.01.2021 16:30


Health, 08.01.2021 16:30