Chemistry, 28.07.2019 13:00 naimareiad
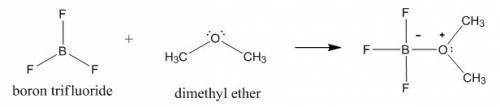
Draw the lewis structure of the 1: 1 adduct that forms in the lewis acid-base reaction between boron trifluoride (bf3) and dimethyl ether ((ch3)2o).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
Draw the lewis structure of the 1: 1 adduct that forms in the lewis acid-base reaction between boron...
Questions


Physics, 12.03.2021 17:30

Mathematics, 12.03.2021 17:30

History, 12.03.2021 17:30

English, 12.03.2021 17:30


Chemistry, 12.03.2021 17:30



Chemistry, 12.03.2021 17:30

Mathematics, 12.03.2021 17:30

Mathematics, 12.03.2021 17:30

English, 12.03.2021 17:30



Mathematics, 12.03.2021 17:30