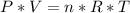Chemistry, 28.07.2019 20:00 heathkid23
If the equation, c2h5oh (l) + 3o2 (g) imported asset 2co2 (g) + 3h2) (l), were performed in a laboratory at room temperature, both of the gas volumes: a)would depend on the surrounding temperature and pressure conditions b)must always be equal because the molar ratios are the same c)must always be equal because the number of representative particles is the same in the equation d)could be calculated without regard to the temperature and pressure surrounding them

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


You know the right answer?
If the equation, c2h5oh (l) + 3o2 (g) imported asset 2co2 (g) + 3h2) (l), were performed in a labora...
Questions


Mathematics, 12.12.2020 20:00

Mathematics, 12.12.2020 20:00





Mathematics, 12.12.2020 20:00



Geography, 12.12.2020 20:00


History, 12.12.2020 20:00

Social Studies, 12.12.2020 20:00

Mathematics, 12.12.2020 20:00


Mathematics, 12.12.2020 20:00

Mathematics, 12.12.2020 20:00


Mathematics, 12.12.2020 20:00