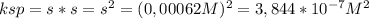(a) at 10°c, 8.9 10−5 g of agcl(s) will dissolve in 100. ml of water. (i) write the equation for the dissociation of agcl(s) in water. (ii) calculate the solubility, in mol l-1, of agcl(s) in water at 10°c. (iii) calculate the value of the solubility-product constant, ksp, for agcl(s) at 10°c. (b) at 25°c, the value of ksp for pbcl2(s) is 1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
(a) at 10°c, 8.9 10−5 g of agcl(s) will dissolve in 100. ml of water. (i) write the equation for the...
Questions






Social Studies, 21.07.2019 21:30

Mathematics, 21.07.2019 21:30



Chemistry, 21.07.2019 21:30

Biology, 21.07.2019 21:30

Spanish, 21.07.2019 21:30


Physics, 21.07.2019 21:30

Advanced Placement (AP), 21.07.2019 21:30



History, 21.07.2019 21:30


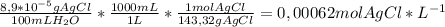
![ksp= [Ag^{+}]*[Cl^{-}]](/tpl/images/0144/2915/6d867.png)