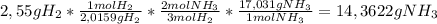Chemistry, 29.07.2019 12:30 lavishbre12
Given the balanced equation 3h2(g)+n2(g)=2nh3(g), calculate the mass of nh3 produced by the complete reaction of 2.55g of h2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 23:00
Need asap question 1 minerals are organic compounds. true false question 2 what vitamin can be found in foods like oranges, grapefruits, and broccoli? a. vitamin a b. vitamin k c.vitamin c d. vitamin d question 3 what are minerals? a. chemical elements that are needed for body processes. b. organic compounds that the body needs in small amounts to function properly. c. small molecules used to build proteins. d. an organic compound that is insoluble in water and includes fats. question 4 how many types of vitamins does the human body need? a. 15 b. 11 c. 13 d. 17 question 5 vitamins are a good source of energy. true false
Answers: 1
You know the right answer?
Given the balanced equation 3h2(g)+n2(g)=2nh3(g), calculate the mass of nh3 produced by the complete...
Questions

English, 25.08.2020 05:01



Arts, 25.08.2020 05:01



English, 25.08.2020 05:01


Mathematics, 25.08.2020 05:01

Mathematics, 25.08.2020 05:01

Mathematics, 25.08.2020 05:01


Mathematics, 25.08.2020 05:01

Biology, 25.08.2020 05:01




History, 25.08.2020 05:01