
Chemistry, 29.07.2019 16:30 damientran
Can someone . a sucrose solution is prepared to a final concentration of 0.170m . convert this value into terms of g/l, molality, and mass % (molecular weight, mwsucrose = 342.296g/mol ; density, ρsol′n = 1.02g/ml ; mass of water, mwat = 961.8g ). note that the mass of solute is included in the density of the solution,

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Can someone . a sucrose solution is prepared to a final concentration of 0.170m . convert this valu...
Questions

Mathematics, 19.03.2021 19:30


Mathematics, 19.03.2021 19:30



Advanced Placement (AP), 19.03.2021 19:30




Mathematics, 19.03.2021 19:30



Mathematics, 19.03.2021 19:30




Mathematics, 19.03.2021 19:30

Mathematics, 19.03.2021 19:30

English, 19.03.2021 19:30

Mathematics, 19.03.2021 19:30

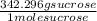 = 58.2 g/L
= 58.2 g/L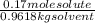 = 0.177 mole / kg
= 0.177 mole / kg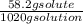 x 100 = 5.7 %
x 100 = 5.7 %

