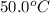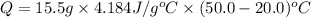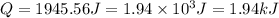Chemistry, 29.07.2019 16:30 Messidapro2687
How much heat is absorbed by 15.5 g of water when its temperature is increased from 20.0°c to 50.0°c? the specific heat of water is 4.184 j/(g°

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
How much heat is absorbed by 15.5 g of water when its temperature is increased from 20.0°c to 50.0°c...
Questions



History, 17.05.2021 21:20

Computers and Technology, 17.05.2021 21:20

Mathematics, 17.05.2021 21:20



Arts, 17.05.2021 21:20

English, 17.05.2021 21:20


Social Studies, 17.05.2021 21:20

Mathematics, 17.05.2021 21:20




English, 17.05.2021 21:20


Geography, 17.05.2021 21:20

Mathematics, 17.05.2021 21:20

Mathematics, 17.05.2021 21:20

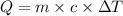
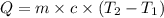
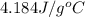
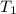 = initial temperature =
= initial temperature = 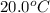
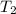 = final temperature =
= final temperature =