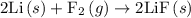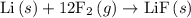For which of the following reactions is δh∘rxn equal to δh∘f of the product(s)? you do not need to look up any values to answer this question. check all that apply. check all that apply. c(s, graphite)+o2(g)→co2(g) li(s)+12f2(l)→lif(s) co(g)+12o2(g)→co2(g) baco3(s)→bao(s)+co2(g) 2li(s)+f2(g)→2lif(s) li(s)+12f2(g)→lif(s)

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2
You know the right answer?
For which of the following reactions is δh∘rxn equal to δh∘f of the product(s)? you do not need to...
Questions






History, 25.07.2019 17:40



History, 25.07.2019 17:40

History, 25.07.2019 17:40

History, 25.07.2019 17:40

Chemistry, 25.07.2019 17:40

Social Studies, 25.07.2019 17:40

Business, 25.07.2019 17:40

Biology, 25.07.2019 17:40

Business, 25.07.2019 17:40

Mathematics, 25.07.2019 17:40

English, 25.07.2019 17:40

History, 25.07.2019 17:40

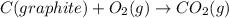
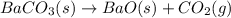
![\Delta H^o_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0148/8328/0ef06.png)
![\Delta H^o_{rxn}=[n_{CO_2}\times \Delta H_f(CO_2)]-[(n_{O_2}\times \Delta H_f(O_2))+(n_f(C)\times \Delta H_f(C))]\\\\\Delta H^o_{rxn}=\Delta H_f(CO_2)-[0+0]=H_f(CO_2)](/tpl/images/0148/8328/a3f93.png)
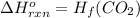
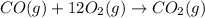
![\Delta H^o_{rxn}=[n_{CO_2}\times \Delta H_f(CO_2)]-[(n_{CO}\times \Delta H_f(CO))]\\\\\Delta H^o_{rxn}=[\Delta H_f(CO_2)]-[\Delta H_f(CO)]](/tpl/images/0148/8328/e48b3.png)
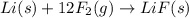
![\Delta H^o_{rxn}=-[12\times \Delta H_f(F_2)]](/tpl/images/0148/8328/fd8a5.png)
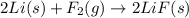
![\Delta H^o_{rxn}=-[\times \Delta H_f(F_2)]](/tpl/images/0148/8328/64079.png)
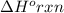 is not equal to
is not equal to 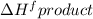 .
.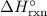 equal to
equal to 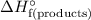 :
:
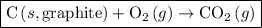
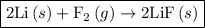
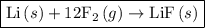
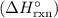 as follows:
as follows:
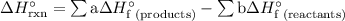 ...... (1)
...... (1)
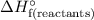 is the standard enthalpy for formation of reactant.
is the standard enthalpy for formation of reactant.
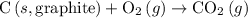
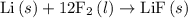
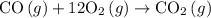
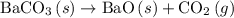
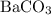 is a compound, not an element. So
is a compound, not an element. So