
Chemistry, 30.07.2019 13:00 chantelljenkins2
Assume that 1.0 mol of c4h10 is completely burned in excess oxygen to form carbon dioxide and water. how many moles of co2 would be produced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1
You know the right answer?
Assume that 1.0 mol of c4h10 is completely burned in excess oxygen to form carbon dioxide and water....
Questions

World Languages, 09.03.2021 22:40



Mathematics, 09.03.2021 22:40

Mathematics, 09.03.2021 22:40

History, 09.03.2021 22:40

Mathematics, 09.03.2021 22:40



Mathematics, 09.03.2021 22:40

Mathematics, 09.03.2021 22:40


Mathematics, 09.03.2021 22:40

Mathematics, 09.03.2021 22:40

Mathematics, 09.03.2021 22:40





Mathematics, 09.03.2021 22:40

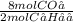 ) = 4 moles of CO₂
) = 4 moles of CO₂

