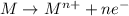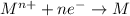Chemistry, 30.07.2019 13:30 onlymyworld27
When an atom in a reactant loses electrons, what happens to its oxidation number? a) its oxidation number decreases b) its oxidation number doubles c) its oxidation number increases d) its oxidation number stays the same

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
When an atom in a reactant loses electrons, what happens to its oxidation number? a) its oxidation...
Questions




Mathematics, 22.07.2019 12:00


English, 22.07.2019 12:00





Mathematics, 22.07.2019 12:00


History, 22.07.2019 12:00


English, 22.07.2019 12:00