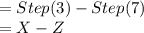Terry, a student, performs a titration. he completes these steps as part of his titration procedure: 1. he cleans and rinses a burette with standardized base. 2. he fills the burette with standardized base solution. 3. he reads and records the initial burette volume. 4. he adds a base from the burette to an acid. 5. he observes a color change in the erlenmeyer flask. 6. he stops the addition of base from the burette. 7. he reads and records the final burette volume. which steps will provide information needed to calculate the volume of base needed to reach the equivalence point? 1 and 6, 3 and 7, 3, 4, and 6, or 1, 2, and 7?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2


Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
Terry, a student, performs a titration. he completes these steps as part of his titration procedure:...
Questions


English, 26.09.2021 21:20

Mathematics, 26.09.2021 21:20

English, 26.09.2021 21:20




Mathematics, 26.09.2021 21:20

Business, 26.09.2021 21:20


Social Studies, 26.09.2021 21:20

Mathematics, 26.09.2021 21:20






Mathematics, 26.09.2021 21:20


![= X\\[/tex ----------------Step (3)Now the final volume in burette after adding the base to the acid solution to attain equivalence point [tex]= Z\\](/tpl/images/0151/0895/41313.png) ----------------Step (7)
----------------Step (7)