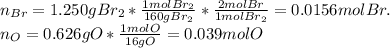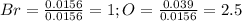Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
Oxides of virtually every element are known. bromine, for example, forms several oxides when treated...
Questions


Computers and Technology, 05.02.2021 15:40

History, 05.02.2021 15:40

Mathematics, 05.02.2021 15:40


Mathematics, 05.02.2021 15:40

Mathematics, 05.02.2021 15:40

Mathematics, 05.02.2021 15:40


Mathematics, 05.02.2021 15:40


Mathematics, 05.02.2021 15:40

English, 05.02.2021 15:40



Mathematics, 05.02.2021 15:40


English, 05.02.2021 15:40

Mathematics, 05.02.2021 15:40

Mathematics, 05.02.2021 15:40

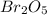

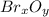 because the grams of bromine are equal before and after the chemical reaction (mass can't be neither created nor destroyed), thus, the bromine grams into the
because the grams of bromine are equal before and after the chemical reaction (mass can't be neither created nor destroyed), thus, the bromine grams into the