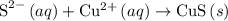Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Identify the spectator ions for the reaction that occurs when aqueous solutions of lithium sulfide a...
Questions

Chemistry, 18.03.2021 20:00

Physics, 18.03.2021 20:00




History, 18.03.2021 20:00

Mathematics, 18.03.2021 20:00

Mathematics, 18.03.2021 20:00


Mathematics, 18.03.2021 20:00


Mathematics, 18.03.2021 20:00


Mathematics, 18.03.2021 20:00

Mathematics, 18.03.2021 20:00

Mathematics, 18.03.2021 20:00

Mathematics, 18.03.2021 20:00


History, 18.03.2021 20:00

Social Studies, 18.03.2021 20:00

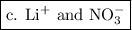 .
.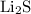 reacts with
reacts with 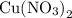 to form CuS and
to form CuS and 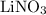 . The balanced molecular equation of the reaction is as follows:
. The balanced molecular equation of the reaction is as follows:
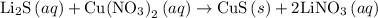
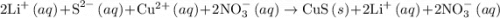
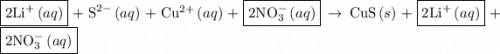
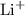 and
and 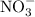 ions are present in the same form on both the reactant and the product side and therefore are known as spectator ions.
ions are present in the same form on both the reactant and the product side and therefore are known as spectator ions.