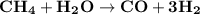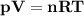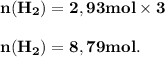Chemistry, 31.07.2019 18:30 katlynnschmolke
The reform reaction between steam and gaseous methane ( ch4 ) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen. suppose a chemical engineer studying a new catalyst for the reform reaction finds that 159. liters per second of methane are consumed when the reaction is run at 294.°c and 0.86atm . calculate the rate at which dihydrogen is being produced. give your answer in kilograms per second. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
The reform reaction between steam and gaseous methane ( ch4 ) produces "synthesis gas," a mixture of...
Questions


History, 20.05.2021 16:30





Computers and Technology, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Chemistry, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30


Mathematics, 20.05.2021 16:30


Mathematics, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Engineering, 20.05.2021 16:30

English, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Social Studies, 20.05.2021 16:30