Chemistry, 31.07.2019 21:00 juansantos7b
Which of the following solutes will lower the freezing point of water the most? the molecular compound sucrose (c12h22o11) the ionic compound magnesium sulfate (mgso4) the ionic compound lithium chloride (licl) the ionic compound calcium fluoride (caf2)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:10
Which equation represents a fission reaction? o "9n+h—150 o 235u + n—190cs + rb+25 o be + he—1c + in o 28 np —> 2390 pute
Answers: 1

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
Which of the following solutes will lower the freezing point of water the most? the molecular compo...
Questions

Geography, 15.12.2020 08:30

Mathematics, 15.12.2020 08:30

Mathematics, 15.12.2020 08:30

English, 15.12.2020 08:30



Mathematics, 15.12.2020 08:30

English, 15.12.2020 08:30

English, 15.12.2020 08:30

Chemistry, 15.12.2020 08:30

Mathematics, 15.12.2020 08:30


Mathematics, 15.12.2020 08:30

Health, 15.12.2020 08:30


Mathematics, 15.12.2020 08:30

Mathematics, 15.12.2020 08:30

Mathematics, 15.12.2020 08:30


Mathematics, 15.12.2020 08:30

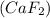
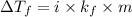
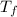 = change in freezing point
= change in freezing point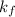 = freezing point constant
= freezing point constant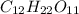 , i= 1 as it is a non electrolyte and does not dissociate.
, i= 1 as it is a non electrolyte and does not dissociate.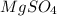 , i= 2 as it is a electrolyte and dissociate to give 2 ions.
, i= 2 as it is a electrolyte and dissociate to give 2 ions.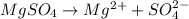
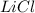 , i= 2 as it is a electrolyte and dissociate to give 2 ions.
, i= 2 as it is a electrolyte and dissociate to give 2 ions.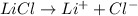
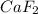 , i= 3 as it is a electrolyte and dissociate to give 3 ions.
, i= 3 as it is a electrolyte and dissociate to give 3 ions.